How to Calculate Acid Neutralizing Capacity
Measurement of Acid Neutralizing Capacity CEE 4530. With this method approximately 05 g.

Measurement Of Acid Neutralizing Capacity
Moles OH- 002 M100 milliliters.
. The terms alkalinity ANC and carbonate alkalinity are used in this manual as follows. The acid neutralization capacity ANC of the studied samples was determined using the modified Sobek et al. Moles OH- 0002 moles.
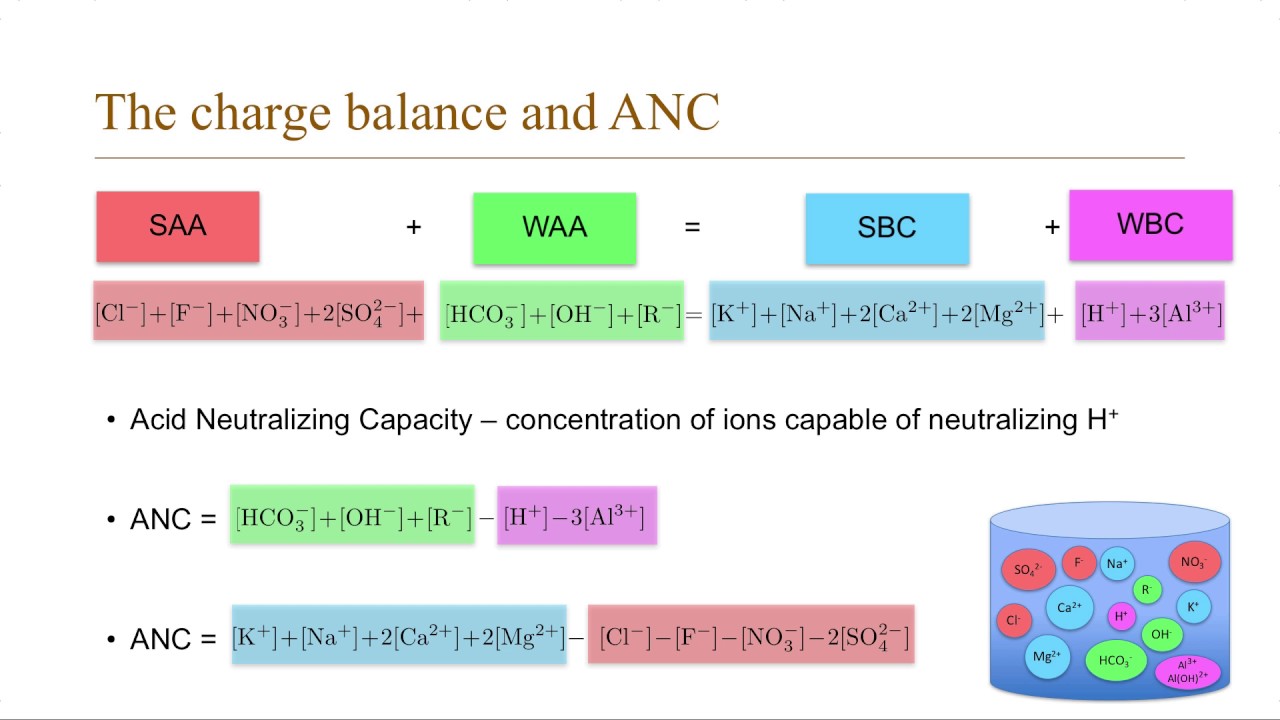
ANC is the acid-neutralizing capacity of solutes plus particu-lates in an unfiltered water sample reported in equivalents per liter or milliequivalents or microequivalents per liter. 10 100 n acid actually neutralized n acid theoretically neutralized. ANC is defined as the difference between cations of strong bases and anions of strong acids see below or dynamically as the amount of acid needed to change the pH value from the samples value to a chosen different.
Using a buret add 500 mL of the acid to one calorimeter and put the plastic cover in place. Using a second buret add 500 mL NaOH to the duplicate calorimeter. Completely neutralize the acid.
PequivL fit straight lines with an error unmeasurable in practice r2 10000. Put the plastic cover in place. Moles OH- 002 M01 liters.
Record the volume of acid. Testing can determine how much acid would need to be added to a quantity of water to change the pH. Alkalinity and ANC provide information on the suitability of water for uses such as irrigation determining the efficiency of wastewater processes determining the presence of.
2 As Or increases the slope of the plots decreases slightly. Procedures of Kjeldahl method. The solution will be titrated with base of known concentration to determine the amount of acid not neutralized by the tablet.
Et s VN V 19 pH Measurements The pH can be measured either as activity H as measured approximately by pH meter or molar concentration H. Nt normality of titrant equivalentsL. Acid neutralizing capacity is a measurement of the buffering abilities in a sample of water.
Alkalinity and the acid neutralizing capacity ANC are deter-mined using identical electrometric procedures involving the aci-dimetric titration of a sample. ANC is equivalent to alkalinity for samples without titratable particulate matter. It is often sold in nurseries and garden stores as a bug killer.
ANC is the acid-neutralizing capacity of solutes plus particulates in an unfiltered water sample reported in milliequivalents or microequivalents per liter. Calculate the number of meq of acid consumed and express the result in terms of meq of acid consumed per g of the substance tested. Acid Neutralizing Capacity Of An Antacid Adding 76 oz or 475 lbs of boric acid per 10000 gallons of water will provide 10 ppm of borate.
Alkalinity determined on a filtered sample and Acid Neutralizing Capacity ANC determined on a whole-water sample are measures of the ability of a water sample to neutralize strong acid. Carbonate alkalinity is the acid-neutralizing capacity attributable to carbonate solutes. P Alkalinity applies to the acid.
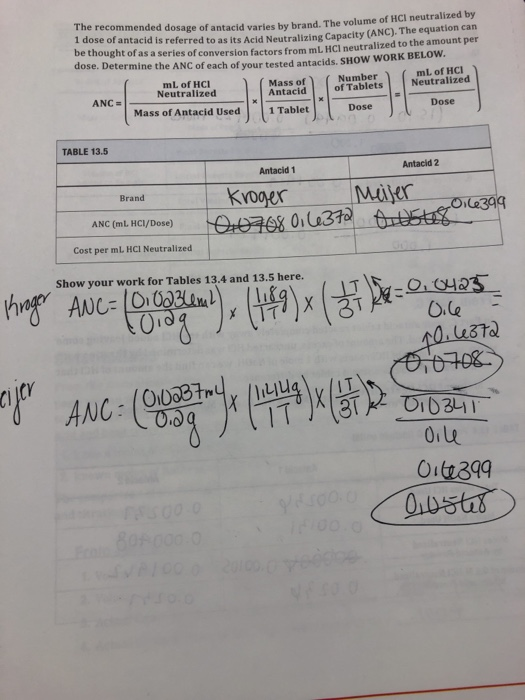
Acid neutralizing capacity per gram of antacid. Therefore the number of moles of HCl that reacted with the antacid should be equal to the total number of moles of HCl minus the number of moles of excess HCl. Acid neutralizing capacity USPNF 2005 The.
Total mEq 30 N HCl V NaOH N NaOH in which N HCl and N NaOH are the normalities of the hydrochloric acid VS and the sodium hydroxide VS respectively. Express this comparison as the ratio of the actual amount of acid that a tablet neutralizes to the theoretical amount that it should neutralize to three significant figures. Acid-neutralizing capacity or ANC in short is a measure for the overall buffering capacity against acidification of a solution eg.
Laboratory Research in Environmental Engineering. The only difference is that the al-kalinity sample is filtered but the ANC sample remains unfil-tered. And V NaOH is the volume of.
Calculate the number of moles of OH-. Measure the temperature of the acid and base at 30-second intervals as specified on Data Sheet 1. 𝐴 𝐶 𝑎 𝑖 𝑎 𝑖 𝑎 𝑎 𝑎 𝑖 MaterialsEquipment Funnel Beakers 250 mL 2 Buret with stand and clamp Erlenmeyer flask 125 mL 2 for filling buret Mortar and pestle Volumetric pipet 2000 mL.
Also Known As Haptics Lab IITM. If it cant buffer very well the pH may drop quickly with exposure to acid. Take this amount and divide by the mass of the sample and you have your acid neutralizing capacity.
Calculate the number of mEq of acid consumed by the formula. Acid-Neutralizing Capacity Certainly this test is applicable only to measure the acid-neutralizing capacity of an antacid tablet. Ns normality in this case alkalinity or ANC of sample equivalentsL.
Moles Molarity x Volume. Volume moles H 0075 Molarity. Surface water or soil water.
Carbonate alkalinity is the acid-neutralizing capacity attrib-. Calculate the Volume of HCl needed. The titration procedure involves incrementally adding known volumes of standardized normality strong acid or base to a known volume of unknown normality base or acid.
To nd the number of moles of acid neutralized by the tablet the number of moles of acid neutralized in the titration is subtracted from the moles of acid in the initial solution. Procedure for Powders Effervescent Solids Suspensions and Other Liquids Lozenges Nonchewable Tablets Chewable Tablets and Capsules Pipet 300 mL of 10 N hydrochloric acid VS into the Test Preparation while continuing to stir with the Magnetic Stirrer. ANC is equivalent to alkalinity for samples without titratable particulate matter.
Moles H moles OH-Volume 0002. Record the volume of base. Slopes are 1000 0980 0971 and 0958 for waters con- taining 0 10 20 and 30 mgL organic acid respectively.
Vs volume of sample liters. How do you calculate the neutralizing power of an antacid. The choice only affects the slope of F 1 since H H γ.
Note Where the acid-neutralizing capacity of the specimen under test is greater than 25 mEq use 600 mL of.
Water Chemistry 3 Charge Balance And Anc Youtube

Acid Neutralizing Capacity Of Antacid Tablets Need Chegg Com

Acid Neutralizing Capacity Of An Antacid
Solved Recommended Dosage Of Antacid Varies By Brand The Chegg Com
No comments for "How to Calculate Acid Neutralizing Capacity"
Post a Comment